U-led group suggests ways to fast-track Ebola vaccines
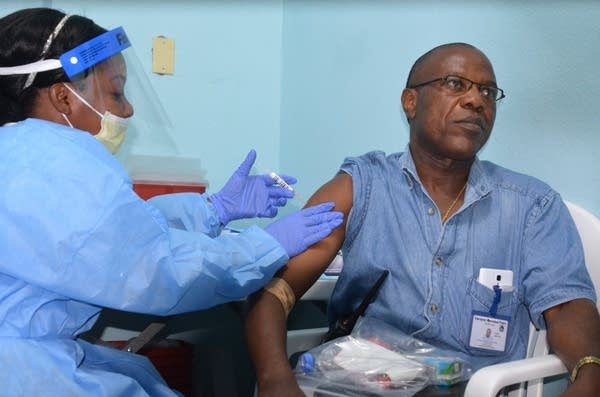
Doctor Francis Kateh volunteers to receive a trial vaccine against Ebola, February 3, 2015. The first large-scale trials of two Ebola vaccines began in Liberia on February 2.
Zoom Dosso | AFP | Getty Images
Go Deeper.
Create an account or log in to save stories.
Like this?
Thanks for liking this story! We have added it to a list of your favorite stories.


